Introduction: Asymptomatic patients with a new diagnosis of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) are usually managed by active observation; however, many patients report elevated levels of distress and worry during the ‘watchful waiting’ phase. Psychological distress may have clinical implications given that natural killer [NK] and T cells are not only sensitive to distress, but also critical in controlling disease progression. Thus, the purpose of this study was to examine associations of psychological functioning and quality of life (QOL) with biomarker expression and functional activity of immune cells from peripheral blood of CLL/SLL patients being managed with surveillance.
Methods: We conducted a multisite, prospective study in patients with indolent B-cell neoplasms who were managed with surveillance. Participants completed measures of perceived stress (Perceived Stress Scale), anxiety and depressive symptoms (Hospital Anxiety and Depression Scale) and quality of life (FACT-G) at study entry and then every 6 months for up to 2 years or disease progression (whichever occurred first). Blood samples were collected at study entry and every 6 months for immune phenotyping using multiparametric flow cytometry (up to 13-color). Biomarkers of activation, viability, proliferation, exhaustion, and receptor expression were assessed on subsets of T, NK, and myeloid cells, and mean fluorescence intensity or percentage of cells staining were determined.
Results: Between April 2016 and November 2020, 233 patients were enrolled at two academic institutions in Philadelphia, including 111 with a diagnosis of CLL/SLL. Of these, 76 patients also had viable samples for immune phenotyping. Participants were predominantly male (60.5%) with a mean age of 63.7 years (SD=9.1 years). Approximately 5% self-reported their race as Black or African American and 4% were of Hispanic ethnicity. At enrollment, the median time since diagnosis was 10.6 months (median follow-up time = 23.6 months).
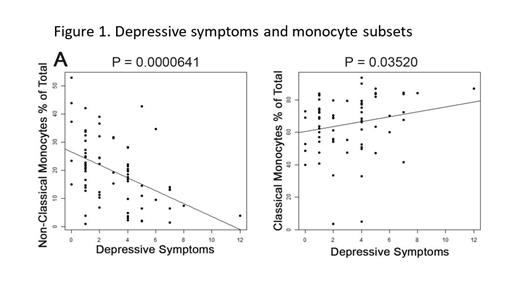
Multivariable regression analyses revealed that increased depressive symptoms were associated with a significant shift from lower percentages of non-classical monocytes (NCM) to higher percentages of classical monocytes (CM; see Figure 1). Similar significant population shifts from reduced NCM toward increased CM were also observed with increased levels of stress and anxiety. These results suggest that patients reporting increased stress, anxiety, and depressive symptoms have fewer of the “patrolling” NCM and increased “sensing” CM in peripheral blood.
We also assessed expression of numerous innate immune receptors, including DNAM-1, which can stimulate responses by NK and T cells toward tumors. We observed lower expression of DNAM-1 on CD4 + helper T cells and CD8 + cytotoxic T cells in patients with increased depressive symptoms (p< 0.05). Similarly, patients reporting lower QOL also expressed lower levels of DNAM-1 on CD4 + and CD8 + T cells, as well as cytotoxic CD56 dim NK cells (p<0.05). As DNAM-1 is important for antitumor responses, our data suggest that patients with higher depressive symptoms have T and NK cells with reduced capacity to respond to CLL/SLL tumor cells. NKp30, another activating receptor on NK and CD8+ T cells promoting cytotoxicity, was also expressed at lower levels on CD56 dim NK cells and T cells in the peripheral blood of patients reporting depressive symptoms. Patients who reported higher levels of depressive symptoms had lower levels of expression of the activation marker CD69 on CD4+ and CD8+ T cells. Finally, longitudinal analyses revealed that higher levels of depressive symptoms predicted lower levels of PD-L1 on CD56 dim NK cells over time ( p = 0.04).
Conclusion: In CLL/SLL patients, psychological functioning is associated with differential patterns of monocyte distribution. Greater depressive symptoms and lower quality of life predicted lower expression of innate immune receptors, such as DNAM-1 on CD4 + helper T cells and CD8 + cytotoxic T cells. Future directions will investigate whether these measures are associated with disease progression or the occurrence of second primary cancers, given the importance of DNAM-1 and NKp30 in immune surveillance for cancers and reactivation of latent viruses. These findings may have direct translational relevance and enable the early identification of patients at-risk for poor outcomes.
Disclosures
Svoboda:Astra Zeneca: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; TG Therapeutics: Research Funding; Atara: Consultancy; ADCT: Consultancy; Merck: Research Funding; BMS: Consultancy, Research Funding; Genmab: Consultancy; Incyte: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; SEAGEN: Consultancy, Research Funding. Cohen:Genentech/Roche: Consultancy, Research Funding; Ichnos: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy; Abbvie: Consultancy; Pfizer: Consultancy; Janssen: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Novartis: Patents & Royalties, Research Funding; Arcellx: Consultancy. Barta:Janssen: Consultancy; Acrotech: Consultancy; Affimed: Consultancy; Daiichi Sankyo: Consultancy. Landsburg:Epizyme: Membership on an entity's Board of Directors or advisory committees; Calithera: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADCT: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; Curis: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Travel funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal